Using administrative data for randomized evaluations
Summary
Administrative data is information collected, used, and stored primarily for administrative (i.e., operational), rather than research, purposes. These data can be an excellent source of information for use in research and impact evaluation. This document provides practical guidance on how to obtain and use nonpublic administrative data for a randomized evaluation. While many of the concepts in this guide are relevant across countries and contexts, certain sections are only applicable to research conducted in the United States.
Introduction
This guide provides practical guidance on how to obtain and use nonpublic administrative data for a randomized evaluation. Administrative data is information collected, used, and stored primarily for administrative (i.e., operational), rather than research, purposes.1 Government departments and other organizations collect administrative data for the purposes of registration, transaction, and record keeping, usually during the delivery of a service. Examples of administrative data include credit card transactions, sales records, electronic medical records, insurance claims, educational records, arrest records, and mortality records. This guide focuses on nonpublic (i.e., proprietary or confidential) administrative data that may be used in an individual-level randomized evaluation.
Context of this guide
Many of the concepts in this guide are applicable across countries and contexts. However, sections pertaining to compliance (particularly HIPAA and specific ethics requirements) are directly applicable only in the United States. Other jurisdictions with similar regulatory contexts may have similar legislation (e.g., the European Union’s General Data Protection Regulation, resulting in the general applicability of concepts across countries.
This guide focuses on the following topics:
- Standard processes for accessing administrative data
- The ethical and legal framework surrounding the use of administrative data for randomized evaluations
- Common challenges in using administrative data
Why use administrative data?
There are a number of advantages to using administrative data for research:2
-
Cost and ease. Using administrative data may be less expensive and logistically easier than collecting new data. Unlike primary data collection, administrative data collection does not require development and validation of a survey instrument, contracting a survey firm or enumerators, or tracking subjects for follow-up.3
- Reduced participant burden. Subjects are not required to provide information to researchers that they have already shared in other contexts.
- Near-universal coverage. Many existing administrative databases provide a near-census of the individuals relevant to a given study. Often, both treatment and control subjects are present equally in these data, as are subjects who may be less likely to respond to follow up surveys for reasons related to their treatment status.
- Accuracy. Administrative data may be more accurate than surveys in measuring characteristics that are complex or difficult for subjects to remember (e.g., income, consumption).4
"An example of the value of administrative data over survey data can be seen in the Oregon Health Insurance Experiment’s study of the impact of covering uninsured low-income adults with Medicaid on emergency room use. This randomized evaluation found no statistically significant impact on emergency room use when measured in survey data, but a statistically significant 40 percent increase in emergency room use in administrative data (Taubman, Allen, Wright, Baicker, & Finkelstein 2014). Part of this difference was due to greater accuracy in the administrative data than the survey reports; limiting to the same time periods and the same set of individuals, estimated effects were larger in the administrative data and more precise” (Finkelstein & Taubman, 2015).
- Minimized bias. Using administrative data that are captured passively, rather than actively reported by individuals or program staff, minimizes the risk of social desirability or enumerator bias.
- Long-term availability. Administrative data may be collected systematically and regularly over time, allowing researchers to observe outcomes for study participants across long spans of time. These long-term outcomes are often the most interesting from both a research and a policy perspective, and may allow researchers to identify impacts that are not present in the short-term. An example of this can be found in Ludwig et al. (2013):
The Moving to Opportunity (MTO) project tested the impact of offering housing vouchers to families living in high-poverty neighborhoods. Using administrative data from tax returns, researchers found that children who were under age 13 when their families moved to lower-poverty neighborhoods had increased rates of college attendance, higher incomes, and lived in lower-poverty neighborhoods later in life. The higher adult incomes yield significantly higher tax payments, which could result in government savings in the long term. These long-term effects are useful in assessing the impact that housing vouchers may have in lifting families out of poverty, but they were not visible in short-term data.
- Cost data. Some administrative data sources are the authoritative data source of cost data, enabling research on public finances or cost-effectiveness analysis. For example, Medicare claims record the exact cost to the public of the health utilization covered by Medicare.
Additional illustrative examples of the uses of administrative data can be found in J-PAL North America’s brief on the Lessons of Administrative Data.
Administrative data also have limitations, challenges and risks, as described in more detail in the following section, “Potential Bias When Using Administrative Data.” Both the Oregon and MTO studies took advantage of both administrative data and survey data, allowing the researchers to study a broad range of outcomes and individuals.5
Potential bias
Administrative data may be more accurate and less susceptible to certain biases than survey data (Finkelstein and Taubman 2015; Meyer and Mittag 2015), but administrative data is not immune to issues of bias and inaccuracy. Bias is of particular concern when using administrative data if being assigned to the treatment group affects the likelihood that an individual appears in, or could be linked to, administrative data.
Differential coverage: observability in administrative data
Differential coverage, the difference in the type or proportion of missing data between treatment and control subjects, can be a problem even when using administrative data. This problem presents itself when members of the treatment and control group are differentially likely to appear in administrative records, or when researchers are differentially likely to be able to link individuals to their administrative records. When this happens, researchers cannot be sure that the treatment and control groups are still statistically equivalent, and impact estimates based on these data may be biased. In these situations, special care must be taken in considering how to treat missing or unmatched outcomes. Attempting to correct for these problems can be problematic, as the types of individuals missing in each group may differ in unobservable ways.
Differential coverage may arise in the following ways when using administrative data:
- Identifiers obtained after enrollment or baseline are used to link individuals to administrative data. For example, members of the treatment group may be more willing to share their Social Security number after several interactions with a member of the study team. Control group members, however, have no such additional trust. This results in researchers having a higher likelihood of finding administrative records for treatment group members than control group members.
- Using data generated by the same program to which participants are randomly assigned. Using program process or monitoring data to evaluate the effects of random assignment to that same program (or encouragement to participate in that same program) is usually not appropriate because treatment group members may be more likely to appear in program implementation data than control group members due to their random assignment status. Further, this data is likely not collected in the same way or with the same accuracy for both the treatment and control group, leading to bias. For example, consider a hypothetical evaluation that assigns some individuals to receive financial counseling at a specific credit union branch. Measuring financial health using data from that same branch would likely capture a much larger proportion of the treatment group--who are now engaged with the branch--than the control group. In this case, researchers may gain a more complete picture of financial health by using data from a national credit reporting agency.
- Members of the treatment group are more (or less) likely than the control group to appear in a certain source of administrative data due to their treatment assignment. Consider the following hypothetical example:
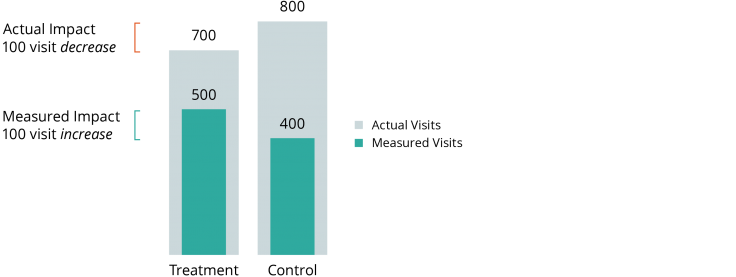
Researchers plan to measure the impact of an intensive home health-care program on frequency of hospital admissions using Medicaid claims data. As a part of their normal services, the home health program also helps participants enroll in social services they may be eligible for, including Medicaid. Because of the assistance offered by the home health program, individuals in the treatment group are more likely to appear in Medicaid records than individuals in the control group. As illustrated below, even if the intervention actually reduces hospitalization (e.g., from 800 to 700), researchers analyzing the Medicaid data may see the opposite effect (e.g., an increase from 400 to 500) due to the larger share of the treatment group’s hospitalizations that appear in the data.
Reporting bias
Many types of administrative data are collected passively, as sales, orders, or transactions occur. However, certain elements within an administrative data set may be actively recorded by a human, rather than passively captured as transactions occur. For example, mothers’ self-reports are the basis for much of the pregnancy history and prenatal care records present on birth certificates. Studies validating birth certificate data have found that these self-reported data have lower validity in comparison with information on birth weight, Apgar score, obstetric age, and methods of delivery, which are recorded based on the observations and measurements of a professional (Lee et. al 2015).
Depending on the original purpose for collecting the data, the organization collecting the data may have differing incentives to verify the accuracy of the information. There may also be incentives for either (or both) the subject and the administrative organization to misreport data. For example, an individual may be incentivized to underreport income in an application for social welfare services. For health-care claims, expensive procedures are likely to be reported in order to receive payment, while some smaller procedures may not be reported if the expected probability of payment is low.
Understanding how and why administrative data is reported, collected and verified is critical to assessing its validity. Further, it is important to understand whether the experiment itself is expected to manipulate incentives relevant to data reporting and validity.
For example, high-stakes testing has been linked to a number of strategies to artificially boost standardized test scores. The administrative records of schools in which substantial score-boosting has occurred may be less accurate measures of student learning than an independent cognitive assessment conducted by a researcher.
Finding administrative data
Implementing partners are an invaluable resource for identifying sources of administrative data relevant to an evaluation. The partner may already have access to certain types of data through their standard work, creating a natural starting point for data discussions. For example, public health nonprofits may have existing partnerships with state Medicaid agencies that can be leveraged to expand an existing data use agreement.
Outside of this channel, J-PAL North America is working to compile a catalog of administrative data sets that may be used in randomized evaluations, focusing on data sets not for public use.
Ideas and examples of administrative data in the US include:
- The Research Data Assistance Center (ResDAC), which provides information and assistance with applying for access to data from the Centers for Medicare and Medicaid Services
- Credit reporting agencies: Equifax, Experian, and TransUnion
- The American Economic Association hosts resources enumerating sources and procedures for accessing US federal administrative data, and non-US data
- Researchers have compiled an inventory of data sets used to study education
- Google’s Dataset Search tool that “enables users to find datasets stored across thousands of repositories on the Web, making these datasets universally accessible and useful.”
Some additional considerations on how to find administrative data by each sector are as follows:
- Health data can be accessed through a regional or national department of health or vital statistics office, by going directly to a hospital or clinic, or through health insurance records.
- Financial data can be sourced from banks, credit unions, and credit reporting agencies.
- Education data can be collected directly from schools or on a larger scale from a country’s ministry of education. For example, J-PAL’s Latin America and Caribbean office and Innovations for Poverty Action’s Peru office are working with Peru’s Ministry of Education on a variety of evaluations to determine the effects of simple interventions on educational outcomes. They’re using some survey data, but are primarily relying on administrative data from the Ministry of Education.
Cost
Administrative data sets range in price and vary in pricing structure. Data providers may charge per individual record, per range of records (e.g., <10,000 records, 10,000–50,000 records, >50,000 records), by file-year, or may change on a cost-reimbursable basis (e.g., by number of hours spent preparing the data request).
Researchers may be able to take advantage of their partner organization’s existing data relationships to acquire data at a lower price, and more quickly through pre-existing channels. For example, a nonprofit organization that offers credit counseling may already have free or discounted access to credit report data in order to perform their credit counseling activities. Researchers may be able to leverage these existing relationships both to lower the price of data needed for an evaluation, and to learn from the data-transfer procedures encountered by the partner organization.
In other cases, researchers may need to make new requests for data solely for research purposes. Prices vary widely: voter registration records in Pennsylvania are $20 per county; credit report data for a sample of 200,000–300,000 individuals for one year are over $5,000; one year of Medicare outpatient records for the full population of enrollees is over $10,000. While the total cost appears very high, the marginal cost per individual may be far less than costs for primary data collection. The ultimate decision should take into account the sample size, the cost and value of administrative data available, and the cost and anticipated response rate of surveying.
Due to the high cost of data, some data providers (notably, the Centers for Medicare and Medicaid Services) require proof of funding and a letter of support from the funding agency prior to finalizing a data use agreement. In order to carry out these requests, research staff likely need access to administrative and financial information on the principal investigator requesting the data.
Researchers must also take into account the amount of time it takes to form a data request, recognizing the (often many) steps of approval that may be required. Some datasets are subject to review by a privacy board (which may be the same as or in addition to an IRB). Frequency and scheduling of board meetings should be incorporated into the project's timeline. Partner organizations and/or academics who already have acquired similar data may be able to assist by explaining the processes they encountered, and potentially by connecting a research team to the relevant data providers.
Understanding the data universe and contents
It is essential to understand the data universe and data contents when planning to use administrative data. A natural first step is to request a data dictionary from the data provider, assuming one is not available online. While some data are very well documented, it is not uncommon for documentation to be incomplete, even for commonly used data sets.
-
Understand the universe from which the data are collected. If appearing in the data set is contingent on some type of program enrollment or measurement (e.g., enrollment in a social service, membership in a credit union), be sure to understand any applicable eligibility criteria. This is crucial to understanding any potential for bias from using the data set, as described in the previous section under "Differential coverage: observability in administrative data."
Consider the universe from which public school attendance data is collected. Students must live in the school district or receive special assignment to the school, and be enrolled in that school. Living in the district may be correlated with income or engagement in local activities. Receiving special assignment may be correlated with parental involvement or ability. Enrollment in a public school, rather than a private school, may be correlated with income, student achievement, parental motivation, or the availability of a school voucher program. Given these factors, it may not be appropriate to study educational achievement using public school records if the intervention is likely to be correlated with remaining in the school district or enrolling in private school.
-
Understand how and why data is recorded. For example, this includes whether data is actively reported by an individual, or collected passively. If actively reported, understand what the incentives are for accuracy, and if any incentives exist for intentional misreporting.
-
Understand the data contents. Even when provided with a data dictionary that describes the contents of a data set, this step often requires iteration with the data provider to clarify the meaning of the data elements in the data set, as variable names or descriptions may be ambiguous.
-
Understand the available identifiers. The identifiers available in the administrative data set (e.g., name, date of birth, Social Security number, Medicaid identification number) will vary by data provider, as will the subset of these identifiers that the provider is willing to share. To ensure that they can match study records on treatment assignment to administrative records, researchers should verify that there is overlap between the identifiers that they have access to prior to random assignment and those present in any administrative data they plan to use. See "Which identifiers to use to link data sets," under the sub-heading "Data Flow," for more information.
While identifying treatment or control status is essential to performing analysis for a randomized evaluation, and other individual-level characteristics may improve the analysis, researchers do not necessarily need direct access to personally identifying information to complete a randomized evaluation. For further information on this process, see the section below on "Formulating a data request."
Ethics
As with any evaluation involving human subjects, randomized evaluations using administrative data must be carried out in accordance with the principles of ethical research. In the US, the three guiding principles are respect for persons, beneficence, and justice. These principles are incorporated as legal statutes in the Federal Policy for the Protection of Human Subjects (the “Common Rule”) and were previously codified in the Belmont Report.
These principles are incorporated into the review performed by Institutional Review Boards (IRBs), and have implications for all types of research, including evaluations that involve only the use of administrative data. For example, respect for persons obliges researchers to consider whether seeking informed consent prior to accessing individuals’ administrative records is necessary and appropriate. While this may not always be necessary, it may be required in some cases. See the section below on "Compliance" and the Appendix on "Informed consent" for more details. Beneficence requires researchers to minimize possible harms, including minimizing the risk of a breach of confidentiality.
The Collaborative Institutional Training Initiative (CITI) provides certifications in Human Subjects Protections and the National Institutes of Health (NIH) offers an online tutorial on Protecting Human Research Participants. These courses provide more information on the principles of ethical research and their practical applications and implications for research. Most IRBs will require one of these courses to be completed by researchers who interact with human subjects or have access to identified data.
Compliance
Research involving administrative data is very likely to be considered human subjects research, and thus subject to review by the Institutional Review Board (IRB) of the researcher’s home institution.6 Beyond the purview of the IRB, an overlapping web of federal laws and regulations, state laws and regulations, and institutional restrictions and procedures governs access, storage and use of administrative data.
Because compliance requirements and definitions of “identifiable” data differ by field, source, and geography, the guidance of an IRB and legal counsel at the researcher’s home institution can be critical in sorting through compliance and reporting requirements. This guide will focus on the US context. Research conducted in any country by US-based researchers is likely subject to a similar level of IRB review based on funding or university policy. As with US-based research, local regulations may apply as well.
Certain types of administrative data contain particularly sensitive information and require additional documentation and compliance prior to their use for research. Some of this is based on protecting the privacy of individuals. For example, most health data in the United States are created or owned by entities that are subject to the Health Insurance Portability and Accountability Act (HIPAA), which mandates additional documentation and protections for data that fall within its scope. Educational data may be subject to the Family Educational Rights and Privacy Act (FERPA), which has special rules to protect the privacy of student records. Criminal and juvenile justice records are also subject to particular rules, which may vary by district. Some requirements are imposed in order to protect proprietary business information, irrespective of individual privacy. For example, an insurer may want to keep their reimbursement rates and contracts private, or businesses may want to guard their trade secrets. Data providers may apply additional restrictions or conditions beyond what the law requires for the researcher to use their administrative data, especially for data considered “sensitive” for any reason.
Personal data of a European Union resident is regulated by the General Data Protection Regulation (GDPR), which applies when data is processed by an individual, a company or an organization located inside and outside the European Union.
Compliance requirements for using administrative data generally depend on how closely the data can be linked to particular individuals and how sensitive the data is. Table 1: Levels of identifiable data gives a very basic overview of the three main “levels” of identifiability. Ultimately, the responsibility for determining the level of identifiability, and the applicable requirements, lies with the data provider and the IRB. Researchers are expected to make a good-faith judgment of the identifiability of data, given their expertise in data analysis. Minimizing the extent to which the researchers themselves handle identified data can provide for a much smoother process of accessing and using administrative data. For information on how to avoid direct contact with identified data, while retaining individual-level data and treatment assignment, see "Data Flow" below.

The remainder of this section describes the permissions and processes for obtaining data at each of three levels of data identification, with additional detail added for health data due to the overlay of HIPAA.
As there is significant overlap between the requirements, it is recommended that the reader use Tables 1 and 2 to determine which level is likely to apply, review Tables 3 and 4 for overview requirements, and read the relevant section for more details.
Research identifiable data
Research identifiable data contain sufficient identifying information such that the data may be directly matched to a specific individual. Usually, such data contain direct individual identifiers, such as names, Social Security numbers, physical addresses, e-mail addresses or phone numbers. However, data that contain a combination of elements (such as date of birth, ZIP code, and gender) may be considered “identifiable” even if they do not contain names, Social Security numbers, or any other single direct identifier.
In general, research involving such data requires active IRB oversight, formal Data Use Agreements (DUAs), robust Data Security measures, and compliance with any additional requirements, such as those imposed by the data provider, or federal or state law.
Additional considerations for health data
Research identifiable health data obtained by certain data providers (e.g., a health plan, health insurer, or health-care provider) are often subject to the specific requirements and restrictions of the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. These data, which are also known as Protected Health Information (PHI), are particularly sensitive. Disclosure of these data is subject to tight restrictions and may pose substantial risks, including legal liability, for the data provider. A list of data types that make a data set “identifiable” under the HIPAA Privacy Rule can be found in Table 2: Levels of identifiable data (HIPAA).
Researchers are advised to confirm whether their implementing partner or the data provider is subject to HIPAA. Some organizations will incorrectly assume they are subject to HIPAA, thus invoking unnecessary review and regulation. For guidance on determining whether an organization is subject to the HIPAA Privacy Rule (i.e., is a Covered Entity), the US Department of Health & Human Services (HHS) defines a covered entity and the Centers for Medicare & Medicaid Services (CMS) provides a flowchart tool.
Because the HIPAA Privacy Rule imposes liability on health data providers when identified data is involved, health data providers may be particularly cautious about how and with whom they share data. The Privacy Rule’s complex nature may make it difficult for health data providers (and researchers) to understand their obligations, causing them to err on the side of caution. Successful research relationships with these data providers are often predicated on a substantial amount of trust and goodwill.
HHS provides a detailed guide to the requirements associated with research identifiable health data and how the HIPAA Privacy Rule applies to research. Resources on HIPAA are provided at the end of this page.
Limited data sets (HIPAA/health only)
Under the HIPAA Privacy Rule, data sets that exclude specific data items, as listed on Table 2: Levels of identifiable data (HIPAA), are called limited data sets. In general terms, limited data sets exclude direct identifiers of individuals of record, or of their relatives, employers, or household members, such as their addresses and Social Security numbers. These data do contain, however, data elements (e.g., birthdate, ZIP code, and gender) that may allow others to make a reasonable guess as to the identity of the subjects.
Compared to research using identifiable data, research using limited data sets may benefit from a simpler process of obtaining the data from the provider and less restrictive oversight. However, this data is still considered Protected Health Information (PHI) and is still much more sensitive than de-identified data.
While the risks associated with limited data sets are lower than those associated with identifiable data, data providers may still face substantial risk if the data is mishandled. Although the HIPAA Privacy Rule imposes fewer restrictions on the sharing of limited data sets than identified data sets, the complexity of the privacy rule may cause providers of health data to err on the side of caution.
De-identified or publicly available data
De-identified data is data that do not contain sufficient identifiers to link to specific individuals with certainty. Relative to research using identified data, research using de-identified data is likely to benefit from a simpler process of obtaining the data and minimal IRB or compliance oversight. The lower risk of re-identification with de-identified data reduces the risk (i.e., legal liability) to data providers of releasing de-identified data. As a result, data providers are less likely to impose additional restrictions or requirements on data requestors.
While it is often necessary to link individual subjects to their treatment status and outcome measures, it may be possible to obtain the necessary individualized detail while avoiding identifiers by having a party other than the researcher (e.g., the data provider or a partner organization) link data sets. A description of this process can be found in the "Data flow" section.
However, even data that do not pertain to individuals at all may be highly classified or confidential; consider the recipe for Coca-Cola or the Google search algorithm.
While HIPAA provides just one of many ways of defining "identifiable" or "de-identified" data, this table may be a useful reference in understanding when a data set is likely to be considered de-identified.
| Data Elements Allowed By HIPAA | De-identified | Limited Data Set | Research Identifiable |
|---|---|---|---|
| Names or initials | X | ||
| Geographic subdivisions smaller than a state | X | ||
| Street address | X | ||
| City, County, and/or Precinct | X | X | |
| ZIP code (5+ digits) | X |
X |
|
| Equivalent geocodes | X | X | |
| ZIP code (3 digits)* *Provided that the geographic unit formed by combining all ZIP Codes with the same 3 initial digits contains >20,000 people |
X* | X | X |
| Dates directly related to an individual | X | X | |
| Year* | X* | X | X |
| Any elements or dates indicative of age >89 *e.g., year of birth indicative of age >89 would not be permitted in a de-identified data set, though years are otherwise permitted |
X | X | |
| Birth date | X | X | |
| Admission or discharge date | X | X | |
| Death date | X | X | |
| Contact information | X | ||
| Telephone and/or fax numbers | X | ||
| Email addresses | X | ||
| Account numbers and codes | X | ||
| Social Security numbers | X | ||
| Medical record numbers | X | ||
| Health plan beneficiary numbers | X | ||
| Account numbers | X | ||
| Certificate/license numbers | X | ||
| Vehicle identifiers and serial numbers, license plate numbers | X | ||
| Device identifiers and serial numbers | X | ||
| URLs or IP addresses | X | ||
| Visual/biometrics | X | ||
| Biometric identifiers | X | ||
| Fingerprints or voiceprints | X | ||
| Full-face photographs or comparable images | X | ||
| Any other unique identifying number, characteristic, or code | X |
Requirement summary tables
| De-identified or Publicly Available Data (HIPAA) | Limited Data Sets (HIPAA/Health Only) | Research Identifiable Data (HIPAA) | |
|---|---|---|---|
| Data Use Agreements* | Process is typically simple or not required | Almost always required. Somewhat less intensive than DUA for identified data. | Almost always required; usually involves significant review. |
| IRB Oversight | Frequently determined exempt by the IRB. | Frequently determined exempt by the IRB. | Likely requires ongoing review by the IRB. |
| Informed Consent or IRB Waiver | If research is determined exempt, not required by the IRB. | ||
| Likely possible to obtain a waiver of informed consent from the IRB as the risk to subjects is low. | Likely possible to obtain a waiver of informed consent from the IRB. More justification may be necessary than obtaining a waiver for de-identified data. | May be possible to obtain a waiver of informed consent from the IRB. Significant justification and proof of secure data protocols is likely necessary to obtain the waiver. |
|
| Individual Authorization for Research (HIPAA) or IRB Waiver | If research is determined exempt, not required by the IRB. | ||
| Likely possible to obtain a waiver of individual authorization as the risk to subjects is low. | Likely possible to obtain a waiver of individual authorization. More justification may be necessary than obtaining a waiver for de-identified data. | May be possible to obtain a waiver of individual authorization. Significant justification and proof of secure data protocols is likely necessary to obtain the waiver. |
|
| Data Security* | Intensive protocols typically are not required by either data provider or IRB. | Intensive protocols typically are required by either data provider or IRB. | Intensive protocols typically are required by both data provider and IRB. |
*This table summarizes requirements based on the identifiability of administrative data. Researchers and data providers may take additional precautions based on the sensitivity of the data that does not necessarily pertain to individuals or identifiability. For example, trade secrets such as recipes do not pertain to individuals, and yet may never be released even under the strictest of data use agreements and data security protocols.
| De-identified or Publicly Available Data (non-HIPAA) | Research Identifiable Data (non-HIPAA) | |
|---|---|---|
| Data Use Agreements | Process is typically simple or not required. | Almost always required; usually involves significant review. |
| IRB Oversight | Frequently determined exempt by the IRB. | Likely requires ongoing review by the IRB. |
| Informed Consent or IRB Waiver | If research is determined exempt, not required by the IRB. | |
| Likely possible to obtain a waiver of informed consent from the IRB as the risk to subjects is low. | May be possible to obtain a waiver of informed consent from the IRB. Significant justification and proof of secure data protocols is likely necessary to obtain the waiver. |
|
| Individual Authorization for Research (HIPAA) | Not applicable for non-health (i.e., non-HIPAA) data. | |
| Data Security* | Intensive protocols typically are not required by either data provider or IRB. | Intensive protocols typically are required by either data provider or IRB. |
*This table summarizes requirements based on the identifiability of administrative data. Researchers and data providers may take additional precautions based on the sensitivity of the data that does not necessarily pertain to individuals or identifiability. For example, trade secrets such as recipes do not pertain to individuals, and yet may never be released even under the strictest of data use agreements and data security protocols.
EU General Data Protection Regulation (GDPR)
The General Data Protection Regulation (GDPR) introduces a major change in the way personal data of an EU resident is processed by an individual, a company or an organization located inside and outside the European Union. The regulation was approved by the EU Parliament in 2016, and came into effect in May 2018. The GDPR aims to regulate the use of personal data by imposing a specific way to ask for individual consent, specifying data subject rights and creating concrete sanctions.
Data processors and controllers must inform and ask consent from an individual about the data they collect and the possible use. The request for consent must be unambiguous and in an intelligible and easily accessible form, explicitly including the purpose for data processing.
The GDPR also introduces:
- The right to access, which allows citizens to question the data controller about their collected personal data (e.g., what kind of information about them has been collected, date and location of collection, purposes, etc.).
- The right to be forgotten, which grants citizens the right to have their data erased, meaning that individuals have more control over their personal data.
- Data portability, which aims to regulate migration of personal data of an individual from one control provider to another under his/her request.
GDPR Resources
- The official GDPR website and FAQs
- The Information Commissioner’s Office (ICO), the agency that will enforce the GDPR in the UK, has created a Guide to the GDPR.
Formulating a data request
In order to extract data from their database, data providers will often need their programmers to write a query to pull the specific data necessary for the research. Researchers should not assume that the programmers have background knowledge about their particular research project, and therefore should write clear and concise data requests that include:
- Timeframe for request, in calendar months/years
- Acceptable or preferable data format (e.g., ASCII, SAS, Stata)
- Data structure (e.g., multiple tables with unique keys for merging versus single, pre-merged data set, long or wide form)
- List of variables requested
- Clarification or notes for each variable
Specify whether identified data or other elements likely to be "sensitive" are necessary. Many data providers will assume you need access to explicit identifiers, such as name or Social Security number, and may stop conversations short based on this understanding. If you do not need access to identified data, be explicit about this, as it will likely speed the process.
Beware of requesting "all of the data." While it may be appealing to request “all” of the data or “as much as possible,” there are dangers with this approach. Without specifying the necessary data elements, researchers may receive less data than they anticipate, or may complete a data use agreement that does not guarantee access to all needed elements. Such a request may also raise red flags with the data provider’s legal team, and create unnecessary hurdles if sensitive data is not truly required.
Further, data sets that are not necessarily sensitive or identifiable on their own may become more sensitive when combined with additional external data sets.
Request specific variables of interest rather than general subjects. For example, researchers may wish to measure the effect of a job training program on the propensity of individuals to hold a “professional” job. Rather than requesting general indicators of having a professional job, researchers should request specific variables, such as job codes and titles. Given a list of specific variables, the data provider may be able to suggest a closely related additional variable of interest: for example, the industry code.
Ensure researchers and data providers understand the data and the request in the same way. Often, researchers make a request for data with a specific project and set of assumptions in mind. Without understanding this background, the data provider may interpret a seemingly straightforward request in a vastly different way.
For example, a request for “all checking transactions” may be interpreted as all transactions that occurred through a written check, or all transactions from the checking account. A phone call or detailed email exchange can help to identify and resolve any problems.
Identify points of contact within the data provider organization. Many data requests are made unnecessarily complicated due to confusion from staff working outside of their normal area of expertise. Successful relationships and requests often involve a high-level owner, director, or policy representative, who understands the general need and importance of the data request and can push the request through any bureaucracy; a data or IT expert who understands the available data elements and would work through the data extraction, matching, and transfer; a programmatic or institutional expert who understands how the data relate to the research questions; and a legal expert who can provide detail on compliance issues and map the pathway to creating a data use agreement.
Leave as little room for interpretation as possible. Request raw inputs rather than calculated or aggregated outcomes. For example, rather than requesting “age”, request the date of service and the date of birth, from which age can be calculated. Rather than requesting “number of transactions above $100,” request the dollar amount of all transactions.
Initial data request:
- Data for the year before and year after the intervention
- Distance from home to school
- Number of children in the household enrolled in school
- Date of birth
- Age
Data provider comments:
"How do you want us to calculate distance? We have an indicator whether there is a school-bus route, and which route it's on. Do you really need both date of birth and age? We assume you want only elementary school records. When is the intervention?"
Updated, refined data request:
- School address
- Enrollment list for all district schools at all levels for the 2014-15 and 2015-16 school years
- Student identifier
- Date of birth
- Student home address
- Bus route (yes/no)
- Bus route code
Data flow
Taking into account any ethical, legal, or resource restrictions, a data flow strategy that maps how data will be linked across administrative, survey, and intervention data should be developed during the design phase of a randomized evaluation. The data flow strategy should include:
- How identifying information will be gathered for the study sample
- Which identifiers will be used to link intervention data and treatment assignment with administrative data
- Which entity or team will perform the link
- What algorithm will be used to link data
- What software will be used to link data
In the simplest case, a data provider may be willing and able to send a fully identified data set directly to the researchers, leaving all other steps of the linking process in the research team’s hands. Often, ethical or legal restrictions will preclude this. The following sections describe data flow strategies for more complex scenarios.
To motivate this section, we will use a hypothetical example in Tables 5-7 where random assignment will take place from an existing list of individuals that includes baseline characteristics, and outcomes data will be collected from two external sources.
| Name | SSN | DOB | Income | State |
|---|---|---|---|---|
| Jane Doe | 123-45-6789 | 5/1/50 | $50,000 | FL |
| John Smith | 987-65-4321 | 7/1/75 | $43,000 | FL |
| Bob Doe | 888-67-1234 | 1/1/82 | $65,000 | GA |
| Adam Jones | 333-22-1111 | 8/23/87 | $43,000 | FL |
| James Trudu | 123-45-9876 | 5/17/60 | $50,000 | FL |
| Joyce Gray | 587-157-8765 | 7/28/80 | $43,000 | FL |
| Philippe Zu | 224-85-6879 | 3/30/80 | $65,000 | GA |
| Alanna Fay | 341-78-3478 | 11/10/79 | $50,000 | FL |
| Name | SSN | Diabetic? |
|---|---|---|
| Jane Doe | 123-45-6789 | Y |
| John Smith | 987-65-4321 | N |
| Bob Doe | 888-67-1234 | N |
| Adam Jones | 333-22-1111 | Y |
| James Trudu | 123-45-9876 | N |
| Joyce Gray | 687-157-8765 | Y |
| Philippe Zu | 224-85-6879 | N |
| Alanna Fay | 341-78-3478 | Y |
| Name | DOB | Own a Car? |
|---|---|---|
| Jane Doe | 5/1/50 | Y |
| John Smith | 7/1/75 | Y |
| Bob Doe | 1/1/82 | Y |
| Adam Jones | 8/23/87 | N |
| James Trudu | 5/17/60 | Y |
| Joyce Gray | 7/28/57 | N |
| Philippe Zu | 3/30/80 | N |
| Alanna Fay | 11/10/79 | Y |
How identifying information will be gathered for the study sample
Researchers and/or their implementing partner may have direct access to a set study sample--including identifying information--through existing administrative data. For example, this may be a list of students in a classroom, existing customers at a bank, physicians in a hospital, or residents of a town with an existing, complete, identified census.
In other cases, researchers may need to proactively identify or recruit a sample that has not been previously defined or identified. For example, this may include new customers at a store, clients at a bank, patients who visit a doctor’s office, or residents of a town for which researchers do not have census information. In this case, identifying information is likely to be obtained proactively through a baseline survey or intake form.
Which identifiers to use to link data sets
Researchers must select which identifiers to use to link data sets based on the set of identifiers available in both the administrative data and program-implementation data. Given that constraint, there are several tips to take into account when deciding which of these identifiers to use to match data sets:
Use only those identifiers available prior to random assignment (e.g., those collected at intake/baseline) to link to participants’ administrative records. Through engagement with individuals through the intervention, it may be possible to obtain more detailed or accurate identifiers for some individuals. If this information is more readily available for treatment group than control group members, using updated information may introduce bias, as described in the sub-section "Differential coverage: observability in administrative data" within the section on "Why use administrative data," above.
Study participants may be hesitant or unwilling to provide sensitive numeric identifiers at intake (e.g., Social Security number, student ID, account numbers). Even if willing, they may not know this information off-hand. While this problem should be balanced across treatment and control groups, as it applies prior to random assignment, a lack of adequate identifiers can reduce effective sample size. Quickly establishing rapport with participants, specifying what information they will be asked to provide, and explaining how the privacy and confidentiality of their data will be protected throughout the course of the study, may help to maximize the number of identifiers that you are able to collect at baseline.
It may be possible to confirm individual’s identifiers, such as SSN or account numbers, through administrative data, provided that participants’ consent is obtained prior to randomization and that the records themselves pre-date the random assignment.
Use numeric identifiers if possible. These are less prone to typos, misspellings or alternative versions than string identifiers (i.e., words or text such as name or address), making them easier to match.
Use identifiers that are not subject to frequent change. Date of birth and SSN do not change with time. In contrast, names are subject to change, especially among women through marriage. Sometimes, unique identifiers that are used as a part of the program or intervention may change over time. For example, participants may be assigned a key tag with a unique code that grants access to library services. If a participant loses the key tag, they may be assigned a new code and key tag during the intervention and evaluation period. Having related records and backup identification methods can insure against this problem.
Which entity should perform the link
Some agencies have experience accommodating requests for data extraction and matching to a specific list of study participants, and are willing and able to perform these matches following a relatively straightforward discussion or agreement with the researchers. Other agencies may be less willing to perform data matches due to fears about confidentiality or legality, or they may be resource-constrained in their technical ability or personnel time. In these situations, researchers may want to suggest creative solutions to providing hands-on assistance with the matching while maintaining confidentiality.
Data agencies may prefer to use their own staff to match administrative records with lists of study participants, or they may allow researchers to do the matching themselves, either on-site or on a secure device provided and monitored by the data provider.
The decision of which entity should perform the match, and which data flow strategy should be chosen, should be made while considering applicable regulations, confidentiality, data security capabilities, and the potential need to maintain the ability to re-identify the data in order to add follow-up data or new data sources.
Data flow strategies
Data flow option one. For a defined sample of specific individuals, researchers may send the data provider a “finder file” that contains participants’ unique study IDs (as assigned by the researcher) and personal identifiers. The data provider will use the personal identifiers in the finder file to select the matching administrative records. The data provider will then strip off all Personally Identifiable Information (PII), and send back the matched data, including the study IDs. When the data is shared in this way, the data provider often requires the researcher to store the finder file and the de-identified analysis file separately, and to agree never to match them.

Using our example, the researcher will send the following finder file to both Data Provider A and Data Provider B. Since Data Provider A only has Social Security numbers (SSN), and B only has dates of birth (DOB), the researcher may alter the finder file for each data provider. In this scenario, the researcher creates the Study ID.
| Heading | Heading | DOB | Study ID |
|---|---|---|---|
| Jane Doe | 123-45-6789 | 5/1/50 | 1 |
| John Smith | 987-65-4321 | 7/1/75 | 2 |
| Bob Doe | 888-67-1234 | 1/1/82 | 3 |
| Adam Jones | 333-22-1111 | 8/23/87 | 4 |
| James Trudu | 123-45-9876 | 5/17/60 | 5 |
| Joyce Gray | 587-157-8765 | 7/28/57 | 6 |
| Philippe Zu | 224-85-6879 | 3/30/80 | 7 |
| Alanna Fay | 341-78-3478 | 11/10/79 | 8 |
Data providers A and B will return the following files:
| Study ID | Diabetic? | Study ID | Own a car? |
|---|---|---|---|
| 1 | Y | 1 | Y |
| 2 | N | 2 | Y |
| 3 | N | 3 | Y |
| 4 | Y | 4 | N |
| 5 | N | 5 | Y |
| 6 | Y | 6 | N |
| 7 | N | 7 | N |
| 8 | Y | 8 | Y |
The researcher will create the following files:
| Treatment Assignment | Study ID | Income | State | Diabetic? | Own a Car? |
|---|---|---|---|---|---|
| Treatment | 1 | $50,000 | Fl | Y | Y |
| Control | 2 | $43,000 | FL | N | Y |
| Treatment | 3 | $65,000 | GA | N | Y |
| Control | 4 | $43,000 | FL | Y | N |
| Treatment | 5 | $50,000 | FL | N | Y |
| Control | 6 | $43,000 | FL | Y | N |
| Treatment | 7 | $65,000 | GA | N | N |
| Control | 8 | $50,000 | FL | Y | Y |
| Name | SSN | DOB | Treatment Assignment | Study ID |
|---|---|---|---|---|
| Jane Doe | 123-45-6789 | 5/1/50 | Treatment | 1 |
| John Smith | 987-65-4321 | 7/1/75 | Control | 2 |
| Bob Doe | 888-67-1234 | 1/1/82 | Treatment | 3 |
| Adam Jones | 333-22-1111 | 8/23/87 | Control | 4 |
| James Trudu | 123-45-9876 | 5/17/60 | Treatment | 5 |
| Joyce Gray | 587-157-8765 | 7/28/57 | Control | 6 |
| Philippe Zu | 224-85-6879 | 3/30/80 | Treatment | 7 |
| Alanna Fay | 341-78-3478 | 11/10/79 | Control | 8 |
In this method, the researcher maintains full control over the crosswalk between the identifying information, the Study IDs, and the random assignment indicator. This ensures that they will have the technical ability to seek additional data sources in the future without relying on continued cooperation from external partners.
However, if the administrative data is very sensitive, the data provider may not feel comfortable with the researcher having access to both the finder file and the de-identified analysis file, because the study ID could be used to re-identify the analysis file. Options two and three detail data flow strategies that address this concern.
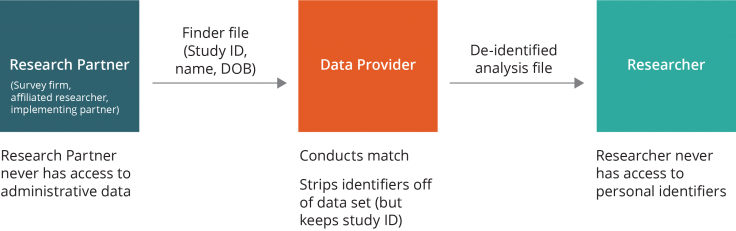
Data flow option two. A close research partner (e.g., an implementing partner or trusted third party) may be made responsible for maintaining the participant list and sending the finder file to the data provider, without ever sharing the identities of the individuals with the researchers. The data provider would conduct the match and then send the de-identified analysis file directly to the researcher. In this scenario, the research partner never has access to the administrative data, and the researcher never has access to the personal identifiers.
If the research partner is willing and able to perform this function, option two is a good fallback for maintaining separation of identified data from outcomes. When considering this option, researchers should consider the strength of the relationship and set realistic expectations for future collaboration. Because the partner, and not the researcher, maintains the identified list of the study sample, the partner’s cooperation would be necessary for any follow-up or long-term evaluations. To ensure that the crosswalk between identifiers and Study ID is maintained, researchers may consider supporting the cost of IT infrastructure and data storage facilities.
The implementer (or third party) will send the following finder file to both Data Provider A and Data Provider B. Since Data Provider A only has Social Security numbers (SSN), and B only has dates of birth (DOB), the partner may alter the finder file for each agency. In this example, the implementer generates the Study ID.
| Name | SSN | DOB | Study ID |
|---|---|---|---|
| Jane Doe | 123-45-6789 | 5/1/50 | 1 |
| John Smith | 987-65-4321 | 7/1/75 | 2 |
| Bob Doe | 888-67-1234 | 1/1/82 | 3 |
| Adam Jones | 333-22-1111 | 8/23/87 | 4 |
| James Trudu | 123-45-9876 | 5/17/60 | 5 |
| Joyce Gray | 587-157-8765 | 7/28/57 | 6 |
| Philippe Zu | 224-85-6879 | 3/30/80 | 7 |
| Alanna Fay | 341-78-3478 | 11/10/79 | 8 |
Data Providers A and B will send the following files to the researcher (and not necessarily to the partner). The partner or implementer will send a similar file including Study ID, Treatment Assignment, Income, and State to the researcher.
| Study ID | Diabetic? | Study ID | Own a car? |
|---|---|---|---|
| 1 | Y | 1 | Y |
| 2 | N | 2 | Y |
| 3 | N | 3 | Y |
| 4 | Y | 4 | N |
| 5 | N | 5 | Y |
| 6 | Y | 6 | N |
| 7 | N | 7 | N |
| 8 | Y | 8 | Y |
The researcher will create an analysis data set as in the previous example. The implementer/partner will create and maintain the crosswalk.
Data flow option three. For very sensitive data, providers may not be willing to release any information that could, hypothetically, be linked to additional characteristics or specific individuals. For example, some jurisdictions protect vital statistics and natality data in this way. These data must be analyzed in isolation, or with only a limited set of additional characteristics approved by the data provider.
The identified list may originate from the researcher, implementer, or a partner. Baseline data may be included in the finder file. The data provider will match the finder file with their data, and de-identify the file to its standards. This may include the removal, censoring, or categorization of certain baseline variables. For example, ages may be converted into ranges, or indicators of low-probability events such as arrest or rare disease may be removed.
In this method, the data provider retains all control over the crosswalk between the finder file and outcomes or administrative data. The data provider may (or may not) maintain this crosswalk internally. If the provider does maintain this crosswalk, they will be able to provide updated administrative data in the future that can be linked to their previous data transfer.
The implementer (or third party) will send the following finder file to both Data Provider A and Data Provider B. Since Data Provider A only has Social Security numbers (SSN), and B only has dates of birth (DOB), the partner may alter the finder file for each agency. In this example, the implementer generates the Study ID.
| Name | SSN | DOB | Income | State | Treatment assignment |
|---|---|---|---|---|---|
| Jane Doe | 123-45-6789 | 5/1/50 | $50,000 | FL | Treatment |
| John Smith | 987-65-4321 | 7/1/75 | $43,000 | FL | Control |
| Bob Doe | 888-67-1234 | 1/1/82 | $65,000 | GA | Treatment |
| Adam Jones | 333-22-1111 | 8/23/87 | $43,000 | FL | Control |
| James Trudu | 123-45-9876 | 3/30/80 | $50,000 | FL | Treatment |
| Joyce Gray | 587-157-8765 | 7/28/57 | $43,000 | FL | Control |
| Philippe Zu | 224-85-6879 | 3/30/80 | $65,000 | GA | Treatment |
| Alanna Fay | 341-78-3478 | 11/10/79 | $50,000 | FL | Control |
In this example, it would not be possible to combine the data from Data Providers A and B. Data Provider A will send the following file to the researcher. Additionally, they may choose to censor the income or geographic data, if they believe it is too specific and potentially identifying.
| Treatment assignment | Income | State | Diabetic? | Own a car? |
|---|---|---|---|---|
| Treatment | $40,000-$60,000 | FL | Y | Y |
| Control | $40,000-$60,000 | FL | N | Y |
| Treatment | >$60,000 | GA | N | Y |
| Control | $40,000-$60,000 | FL | Y | N |
| Treatment | $40,000-$60,000 | FL | N | Y |
| Control | $40,000-$60,000 | FL | Y | N |
| Treatment | >$60,000 | GA | N | N |
| Control | $40,000-$60,000 | FL | Y | Y |
The data provider may maintain the crosswalk.
Data flow option four. Some agencies may not have the capacity to extract and match data for researchers or the ability to freely share a fully identified data set. It may be possible for a researcher to conduct or assist with the match on-site at the data provider, under the supervision of provider staff, and leave with only a de-identified data set.
This may be an informal process, or the researcher may sign forms to act as a volunteer employee of the data provider for a day or more, or sponsor an assistant seated at the data provider. The provider may wish to restrict the researcher’s access to the internet or storage devices in order to prevent the release of confidential data during the match. This approach reduces the burden on the data provider while maintaining a high level of confidentiality.
In this case, the researcher may or may not be permitted to retain a crosswalk between identifiers and the Study ID. The data provider may analyze the resulting de-identified data set and request further action, as in option three.
This option allows the researcher to maintain greater control over the matching algorithm. However, while the burden of data matching is lifted off of the data provider, the researchers must invest in travel, and may have a very limited timeframe to complete the linkage and solve any issues that may arise.
Algorithms for linking data
In general, there are two types of matching strategies: “exact” (deterministic) and “fuzzy” (probabilistic). Regardless of whether the researcher, the data provider, or a third party is performing the match, it is important to understand and document the matching strategy. Using an exact matching strategy minimizes the number of false positives (i.e., a “match” is found, but is in fact not the same individual), but may maximize the number of false negatives (i.e., a “match” is not found, but the same individual is present in both files).
- Exact matching. With “exact” matching strategies, specific identifiers in the finder file are matched to the identifiers in the administrative data sets. If there are minor discrepancies (e.g., reversed month and day in date of birth, or typos in the last name), records will not be identified as a match even though they may be.
- Probabilistic, or fuzzy matching. With fuzzy matching strategies, a more sophisticated algorithm is used to account for the fact that identifiers in the finder file may not match exactly to those in the administrative data sets, but may be close enough to be considered a good match. For reproducibility and transparency, the algorithm or protocol for determining a fuzzy match should be explicitly stated and followed. Manually reconciling data sets and making judgment-based decisions is not a reproducible method.
For example, when linking two data sets, which records are identified as “matches” depends heavily on the variables that are used for the match, and the types and amounts of variances allowed for in a fuzzy match.
| Record | Name | SSN | DOB |
|---|---|---|---|
| A | Jane Doe | 123-45-6789 | 5/1/1950 |
| B | Jonathan Smith | 987-65-4321 | 7/1/1975 |
| C | Bob Doe | 888-67-1234 | 1/1/1982 |
| D | Adam Jones | 333-22-1111 | 8/23/1987 |
| E | Maria Anna Lopez | 532-14-5578 | 10/15/1965 |
| F | Sarah Franklin | 333-22-1111 | 8/23/1987 |
| Record | Name | SSN | DOB |
|---|---|---|---|
| 1 | Jane Doe | 123-45-6789 | 1/5/1950 |
| 2 | John Smith | 987-65-4321 | 7/1/1975 |
| 3 | Bob Doe | 888-67-1243 | 1/1/1982 |
| 4 | Adam Jones | 8/23/1987 | |
| 5 | M. Anna Lopez | 532-14-5578 | 10/15/1965 |
| 6 | Sarah Franklin | 333-22-1111 | 8/23/1987 |
| Exact Match | ||||
|---|---|---|---|---|
| Records | Fuzzy Match | On SSN Only | On Name, SSN, DOB | On Name, DOB |
| A+1 | Could be matched | Match | No match | No match |
| B+2 | Could be matched | Match | No match | No match |
| C+3 | Could be matched | No match | No match | Match |
| D+4 | Could be matched | No match | No match | Match |
| E+5 | Could be matched | Match | No match | No match |
| F+6 | Matched | Match | Match | Match |
Depending on the structure of the data and the treatment of non matched records, the matching errors may decrease the precision of impact estimates. Under some circumstances, the (lack of) balance between the rate of false positives and false negatives may even bias the estimates. For example, consider the case of matching a specific set of individuals with their arrest records. Because arrest records only contain those individuals who were arrested, there is no file that will confirm whether an individual was not arrested, so the researcher would likely attribute a value of “no arrests'' to any records that do not have a positive match with arrest records. Relying on an exact-match strategy will minimize the risk that crimes are falsely attributed to individuals who did not commit crimes. However, false negatives may lead to individuals who did commit crimes being attributed a clean slate. Though this type of matching error may be random across treatment and control, it may still lead to bias and/or to decreased precision in impact estimates (Tahamont et al. 2015; Dynarski et al. 2013).
Consider researchers who have the following list of program participants:
| Record | Name | SSN | DOB |
|---|---|---|---|
| A | Jane Doe | 123-45-6789 | 5/1/1950 |
| B | Jonathan Smith | 987-65-4321 | 7/1/1975 |
| C | Emer Blue | 547-94-5917 | 8/15/00 |
| D | Amy Gonzalez | 431-54-9870 | 12/2/89 |
| E | Bob Doe | 888-67-1234 | 1/1/1982 |
| F | Adam Jones | 333-22-1111 | 8/23/1987 |
| G | Maria Anna Lopez | 532-14-5578 | 10/15/1965 |
| H | Sarah Franklin | 333-22-1111 | 8/23/1987 |
They receive the following arrest records from the state criminal justice agency:
| Record | Name | SSN | DOB | Arrests |
|---|---|---|---|---|
| 1 | Jane Doe | 123-45-6789 | 1/5/1950 | 5 |
| 2 | John Smith | 987-65-4321 | 7/1/1975 | 3 |
| 4 | Amy Gonzalez | 431-54-9870 | 12/2/89 | 2 |
| 6 | Adam Jones | 8/23/1987 | 9 | |
| 7 | M. Anna Lopez | 532-14-5578 | 10/15/1965 | 2 |
Depending on which matching strategy is used, a clean arrest record may be attributed to an individual, even if they were, in fact, arrested:
| Name | On Name only | SSN only | On Name, SSN, DOB | On Name, DOB |
|---|---|---|---|---|
| Jane Doe | 5 | 5 | 0 | 0 |
| Jonathan Smith | 0 | 3 | 0 | 0 |
| Emer Blue | 0 | 0 | 0 | 0 |
| Amy Gonzalez | 2 | 2 | 2 | 2 |
| Bob Doe | 0 | 0 | 0 | 0 |
| Adam Jones | 9 | 0 | 0 | 9 |
| Maria Anna Lopez | 0 | 2 | 0 | 0 |
| Sarah Franklin | 0 | 0 | 0 | 0 |
Software for data linkage
There are several options that may be used for computing a probabilistic (“fuzzy”) match. Stata’s “reclink” provides one option that may be familiar to social-science researchers. Software programs that specialize in matching include Merge Toolbox and Link Plus. Other researchers have used Elastic Search and Open Refine (formerly Google Refine).
Appendices
Consent and authorization
Consent and authorization are related topics, and both are reviewed by Institutional Review Boards or Privacy Boards. While authorization may be obtained during the informed-consent process, consent and authorization are not substitutes.
Informed consent
Informed consent is a process by which research subjects are informed of the research procedures, goals, risks, and benefits, and consent to participate voluntarily. The Common Rule (45 CFR 46.116) and institutional policy dictate certain elements of informed consent. The US Department of Health & Human Services also maintains a tips sheet for informed consent.
Only individuals who are legally adults may give consent. While parents may give consent for their children, the IRB may determine that researchers obtain the assent of the children to participate in the research, in addition to the consent of their parents.
Data providers may want to review the study’s informed consent form to ensure it accurately describes to prospective study participants what data from their organization will be shared and with whom, when the data will be released, and how it will be protected. The organization’s review may result in changes to the study’s informed consent form, which must be approved by an IRB. Researchers who request administrative data for a previously consented study sample may be required to re-obtain for each participant before a data provider will agree to release his/her administrative records (Lee et al. 2015).
For more information, see our resource on defining intake and consent processes, and the resources at the bottom of this page.
Authorization for research (HIPAA)
An authorization is a signed record of an individual’s permission to allow a HIPAA Covered Entity to use or disclose their Protected Health Information (PHI).The authorization must describe the information requested and the purpose, and must be written in plain language that is readily understood by the individual. This is similar to the concept of Informed Consent, and is often embedded within an informed-consent document. However, an authorization has a distinct set of criteria and may be a separate written agreement obtained outside of the informed-consent process.
For more information, see the resources at the bottom of this page.
Last updated September 2021.
These resources are a collaborative effort. If you notice a bug or have a suggestion for additional content, please fill out this form.
Data use agreements
A Data Use Agreement (DUA) documents the terms under which a data provider shares data with a researcher’s home institution for use by the researcher. This agreement, which typically must be approved by legal counsel at the researcher’s home institution, contains a number of provisions that can significantly impact the underlying research. Many universities have a standard template that includes terms and conditions that are acceptable to the university, and were created with researchers’ needs in mind. Using a pre-vetted template may simplify the review process at the institution that created the template. A US-based initiative among 10 federal agencies and 154 institutions (including MIT and other leading universities) created a template DUA. While most institutions prefer using their own template, many member institutions have agreed to use this template as a fallback option.
Described below are certain items commonly found in DUAs that are particularly important for researchers to understand when negotiating with a data provider:
- Review periods and academic freedom to publish. Data providers may request the right to review the research manuscript prior to publication, either to preview the results or to identify the inadvertent disclosure of confidential information. Such review may be acceptable so long as the researcher retains the final freedom to publish at their sole discretion, after reviewing any data provider comments in good faith. Researchers may also want to set reasonable limits on the time allotted to review prior to publication. This provision is important, as providers may attempt to suppress unfavorable results.
- Limits on the publication of summary statistics. Some data providers may impose strict cutoffs for the number of individuals in a “cell” or disaggregated summary statistics. Researchers should be aware of these limits and be sure they are reasonable.
- Individual liability. DUAs are typically entered into by the data provider and the researcher’s home institution and should not personally expose the researcher to the risk of lawsuit if the agreement is breached. Researchers should take note of any forms or addenda requiring individual signatures and consult with their legal counsel prior to signing.
- Destruction or return of the data. Data providers typically require that researchers return or destroy personally identifiable information (or all information) after a particular period of time. Researchers should be sure the time allotted is sufficient to cover the research, publication, and academic journal review process, taking into account whether identifiers may be necessary to link with additional data obtained in the future, or whether data may need to be reviewed at a later date.
Below, common elements and tips for negotiating a DUA are described:
- Data description. While some data providers require a detailed list of data elements requested, other will accept a general description of the data. A general description may allow additional elements to be added more easily without an official amendment, though the researcher may not be ensured access to all necessary elements.
- Data timeframe. Many data providers allow researchers to request several years of data prospectively; others will require an amendment each year to request new data.
- Frequency and time schedule of data transfers. The first data extraction and transfer may take more time and require more communication and iteration than subsequent data pulls. It can be helpful to schedule and plan a “practice” or “sample” data transfer.
- Research subject privacy. Data providers may require the researchers to agree not to use the data within the data set to contact the subjects, their relatives, or obtain additional information about them (i.e., conduct a “follow-back” investigation).
- Personnel. Some data providers require a list of each individual who will have access to the data, while others allow principal investigators to identify “classes” of individuals (e.g., students or employees) who will have access. Identifying classes rather than specific individuals may reduce administrative burden by minimizing the need for future amendments when staff are added or removed from a project.
In addition to the elements described above, DUAs may require researchers to provide a study protocol, and typically contain provisions related to Data Security, confidentiality, IRB or Privacy Board review, the re-release of data, and the allocation of the liability between the data provider and the researcher’s home institution. For more information on describing data security within the context of a DUA, see the Example language for describing a data security plan within our separate resource on Data Security, and the resources at the end of this page.
Timeline
Gaining access to administrative data is a multifaceted process that should be initiated during the design phase of any research project. The time required to establish a data use agreement can vary widely, and depends on the data provider’s capacity to handle such data requests, the sensitivity of the data, and the levels of review that must be undertaken by both sides before a legal agreement can be signed. In a 2015 analysis of data acquisition efforts with 42 data agencies, the MDRC found that it typically takes 7 to 18 months from initial contact with a data provider to the completion of a legal agreement.7
Much of this time is spent in a tandem process of obtaining both IRB and legal approval for a data request. Both processes can involve lengthy review periods, and changes made by one entity must be reviewed and approved by the other. IRBs often require researchers to furnish signed DUAs before approving a study protocol, and data providers often require IRB approval before signing a DUA. Research teams may request provisional approval from one party, making clear to all parties the process and constraints, to find a path forward. Research teams can facilitate the process by proactively and frequently communicating with the IRB and data partners.
Many administrative data sets are available on a lagged basis, to allow time for data entry and cleaning. Beyond this planned data lag, researchers should plan to allow additional time for the data provider to extract and transfer data. This may take several weeks or months, depending on the provider’s capacity and workload. Especially in the first transfer, researchers should expect to iterate with the data provider on matching strategies and data definitions.
Having clear specifications in the data use agreement or memorandum of understanding may help to set expectations between the data provider and the researcher regarding extraction date(s) and frequency.
Encouraging data provider cooperation
Additional incentives may encourage the data provider to cooperate and prioritize a request for administrative data. Depending on the frictions preventing cooperation, incentives may range from a letter of support from a high-ranking official within the data provider’s organization, monetary compensation for staff time and IT resources, or in-kind assistance through providing data analysis relevant to the provider’s needs. To solve issues of trust, researchers have sought co-investigators from within a data provider’s organization and brought on consultants who the data provider organization trusts or who previously worked for the provider.
Data providers may have access to a wealth of data, but they may not have analytics as their primary objective. For example, credit unions, government departments of health and education, and school systems may have access to data that are extremely useful for researchers. However, these organizations are focused on providing services, and may have very small (or nonexistent) data analysis teams. Supplying the provider with relevant and interesting analyses of their own data on an ongoing basis may be very valuable, and may help them prioritize the researcher’s needs.
A more intensive approach is to offer an intern or research assistant who could work directly for the provider. The research assistant’s role would be to assist with the data extraction for the randomized evaluation, but also to assist the provider in other ways. When offering this type of support, it’s best to concretely define and explain how the provider could benefit from the research assistant’s time, and how this would be a logistically viable solution for the provider (e.g., researchers may offer to conduct background checks, oversee the onboarding process and payments, etc.).
We are grateful to Julia Brown, Manasi Deshpande, Jonathan Kolstad, Megan McGuire, Kate McNeill, Adam Sacarny, Jim Shen, Marc Shotland, Kim Smith, Daniel Prinz, Elisabeth O’Toole, Evan Williams, and Annetta Zhou for their insightful feedback and advice. We thank Mary Ann Bates, Stuart Buck, Amy Finkelstein, Daniel Goroff, Lawrence Katz, and Josh McGee for recognizing the need for this document, and for their ideas and inspiration along the way. Alison Cappellieri and Betsy Naymon copyedited this document. Alicia Doyon and Laurie Messenger formatted the guide, tables, and figures. This work was made possible by support from the Alfred P. Sloan Foundation and the Laura and John Arnold Foundation. Any errors are our own.
Disclaimer: This document is intended for informational purposes only. Any information related to the law contained herein is intended to convey a general understanding and not to provide specific legal advice. Use of this information does not create an attorney-client relationship between you and MIT. Any information provided in this document should not be used as a substitute for competent legal advice from a licensed professional attorney applied to your circumstances.
Please send comments, questions, or feedback to [email protected].
ADR UK (Administrative Data Research United Kingdom) gives further definitions and examples of administrative data and its use in research.
For more detail on those listed here, see Finkelstein and Taubman (2015).
This is not to say that administrative data are necessarily cheap; it is not uncommon to see data priced around $10,000 per file.
In Meyer and Mittag (2015), the researchers compared administrative data from SNAP (food stamps), Temporary Assistance for Needy Families (TANF), General Assistance, and subsidized housing from New York State to the Current Population Survey (CPS), which is conducted by the US Census Bureau. They find that income is significantly underreported in the CPS when compared with administrative records, and argue that the choice of data source sharply alters measurements of well-being and the effects of transfer programs.
While a study may use both surveys and multiple sources of administrative data, each outcome should be measured using the same source of data and the same method for both the treatment and control group.
There are very limited exceptions to this rule, and the exceptions may vary by institution. Check with your IRB for details.
See Lee et al. (2015), especially Figure ES.1 Data Acquisition Process: Typical Length of Time to Complete Each Step in The Mother and Infant Home Visiting Program Evaluation-Strong Start report.
For a review, see “The Re-Identification Of Anonymous People With Big Data.”
Additional Resources
The Administrative Data Research Partnership supports administrative data research in the UK.
The “Cheaper, Faster, Better: Are State Administrative Data the Answer?” report provides an overview of working with administrative vital records and Medicaid data in the Mother and Infant Home Visiting Program Evaluation–Strong Start (MIHOPE-Strong Start) project. As such it provides an excellent overview of all steps in the administrative data process.
Harvard University’s Research Data Security Policy classifies data according to five levels of sensitivity and defines data security requirements that correspond to each sensitivity level.
US Department of Health & Human Services’ Guidance Regarding Methods for De-identification of Protected Health Information in Accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule.
The National Institutes of Health’s “How Can Covered Entities Use and Disclose Protected Health Information for Research and Comply with the Privacy Rule?”
45 CFR 164.514 – Describes the HIPAA standard for de-identification of protected health information (original text).
The US Department of Health & Human Services provides a detailed guide to the requirements associated with research identifiable health data and how the HIPAA Privacy Rule applies to research and a guide to understanding HIPAA for all types of users.
45 CFR 164.502 – Uses and disclosures of protected health information (original text).
NIH guidance on complying with HIPAA, including de-identified health information, Authorizations, and Authorization waivers.
45 CFR 164.514 – Describes the HIPAA standard for de-identification of protected health information (original text).
45 CFR 160.103 – Defines Individually identifiable health information.
HIPAA defines a Minimum Necessary Requirement: Covered entities and business associates must make reasonable efforts to limit disclosures of protected health information to the minimum necessary to accomplish the intended purpose of the use, disclosure, or request. Though a limited data set is permitted to contain, for example, an individual's exact birthdate, the birthdate should only be included if it serves a specific research purpose.
CMS and ResDAC define limited data sets and research identifiable files slightly differently by excluding most dates from limited data sets.
45 CFR 164.502 – Uses and disclosures of protected health information (original text).
45 CFR 164.508 – Uses and disclosures for which an authorization is required (original text). HIPAA regulations pertaining to authorizations for the release of health information, and requirements of the authorization.
NIH guidance on complying with HIPAA, including Authorization for research and waivers of Authorizations.
The US Department of Health & Human Services’ guide to understanding the HIPAA Privacy Rule’s relationship to research includes descriptions of the specific requirements of an Authorization for research.
45 CFR 46.116 – Common Rule requirements for informed consent (original text). Paragraph (a) describes the basic, required elements of informed consent; (b) describes additional elements that may be included or required depending on the study; (c) and (d) describe the conditions under which IRBs may waive or approve an alteration of informed consent.
The US Department of Health & Human Services maintains a tips sheet.
MIT’s Committee on the Use of Humans as Experimental Subjects (COUHES) provides template forms and instructions for obtaining informed consent and authorization.
J-PAL researchers can reference checklists and resources on Google Drive.
IPA researchers can reference checklists and resources on Box.
The University of Colorado’s IRB provides examples and discussion of waivers of informed consent.
The Massachusetts Institute of Technology’s Committee on the Use of Humans as Experimental Subjects (COUHES) provides examples of what various types of application forms might look like, and includes instructions.
COUHES also has an investigator quick guide overviewing when review is needed, and guidelines for various procedures in the IRB review process.
The University of Colorado has several guidance documents relating to all elements of the IRB process.
The US Department of Health & Human Services presents decision charts for understanding IRB requirements.
J-PAL researchers can reference the Human Subjects section of Google Drive for checklists and resources including information on protocol, IRB documents, and templates (J-PAL internal resource).
Innovations for Poverty Action (IPA)’s IRB publishes FAQs and resources on their website.
J-PAL North America is developing a Catalog of Administrative Data Sets for use in randomized evaluations.
Medicare and Medicaid data: The Research Data Assistance Center (ResDAC) at the University of Minnesota provides explanation and assistance with applying for access to data from the Centers for Medicare and Medicaid Services.
Credit reporting agencies: Equifax, Experian, TransUnion.
US Federal data: The American Economic Association hosts resources enumerating sources and procedures for accessing US federal administrative data.
Education data: Researchers have compiled an inventory of data sets used to study education.
J-PAL’s resources on working with data, which includes additional resources for VeraCrypt.
For researchers at MIT, Dr. Micah Altman, Director of Research at MIT Libraries regularly presents talks on Managing Confidential Data.
MIT’s Information Systems & Technology Department (MIT internal resource) provides resources on:
-
Secure Shell File Transfer Protocol: SecureFX
-
Encryption (including software recommendations) and whole-disk encryption
Harvard University’s Research Data Security Policy (HRDSP) is an excellent resource for security level classification and security requirement examples.
J-PAL's Research Protocol Checklist
J-PAL and IPA provide sample code to:
The National Institute of Standards and Technology’s (NIST) paper on De-Identification of Personal Information, and explained in their presentation on Data De-Identification.
The National Institute of Standards and Technology’s (NIST) revised guidelines for passwords, explained in more approachable language in a NIST staff blog post.
Several institutions provide guidance on developing data security plans, and describing the plans for grant proposals or data use agreements. Resources include:
- Inter-university Consortium for Political and Social Research’s (ICPSR) Framework for Creating a Data Management Plan and Guidelines for Effective Data Management Plans.
- MIT Libraries’ guide to Writing a Data Management Plan.
- NC State University Libraries’ Data Management Plan Examples.
- Rice Research Data Team’s resource for Developing a Data Management Plan.
- UNC Carolina Population Center’s tools and resources on Security Plans for Restricted-Use Data.
- University of California Curation Center’s Data Management Plan Tool (DMPTool) enables users to organize data management plans according to templates, for example, to adhere to funding requirements. This resource is subscription-based. Please refer to the list of DMP Participants to see if your university or institution already enables an institutional sign-in.
MIT has sample data use and nondisclosure agreements here.
References
Berlee, Anna. 2015. “Using NYC Taxi Data to identify Muslim taxi drivers.” The Interdisciplinary Internet Institute (blog). Accessed November 30, 2015.
Dynarski, Susan, Steven Hemelt, and Joshua Hyman. 2013. “The Missing Manual: Using National Student Clearinghouse Data to Track Postsecondary Outcomes.” w19552. Cambridge, MA: National Bureau of Economic Research. http://www.nber.org/papers/w19552.pdf.
Goodin, Dan. 2014. "Poorly anonymized logs reveal NYC cab drivers’ detailed whereabouts." Ars Technica. Accessed November 30, 2015. http://arstechnica.com/tech-policy/2014/06/poorly-anonymized-logs-reveal-nyc-cab-drivers-detailed-whereabouts/.
Finkelstein, Amy, and Sarah Taubman. 2015. “Using Randomized Evaluations to Improve the Efficiency of US Healthcare Delivery.” http://www.povertyactionlab.org/publication/healthcaredelivery/reviewpaper.
Lee, Helen, Anne Warren, and Lakhpreet Gill. 2015. “Cheaper, Faster, Better: Are State Administrative Data the Answer?” OPRE Report 2015-09. The Mother and Infant Home Visiting Program Evaluation-Strong Start Second Annual Report. MDRC. http://www.mdrc.org/sites/default/files/MIHOPE-StrongStart-2yr_2015.pdf.
Ludwig, Jens, Greg J Duncan, Lisa A Gennetian, Lawrence F Katz, Ronald C Kessler, Jeffrey R Kling, and Lisa Sanbonmatsu. 2013. “Long-Term Neighborhood Effects on Low-Income Families: Evidence from Moving to Opportunity.” American Economic Review 103 (3): 226–31. doi:10.1257/aer.103.3.226.
Mangan, Katherine. 2010. “Chapel Hill Researcher Fights Demotion after Security Breach.” Chronicle of Higher Education. Accessed March 7, 2018. https://www.chronicle.com/article/chapel-hill-researcher-fights/124821/.
Meyer, Bruce, and Nikolas Mittag. 2015. “Using Linked Survey and Administrative Data to Better Measure Income: Implications for Poverty, Program Effectiveness and Holes in the Safety Net.” w21676. Cambridge, MA: National Bureau of Economic Research. http://www.nber.org/papers/w21676.pdf.
Narayanan, Arvind, and Vitaly Shmatikov. 2008. “Robust De-Anonymization of Large Sparse Datasets.” presented at the Proceedings of 29th IEEE Symposium on Security and Privacy, Oakland, CA, May. https://ieeexplore.ieee.org/document/4531148.
Pandurangan, Vijay. 2014. "On Taxis and Rainbows: Lessons from NYC’s improperly anonymized taxi logs." Medium. Accessed November 30, 2015. https://medium.com/@vijayp/of-taxis-and-rainbows-f6bc289679a1.
Tahamont, Sarah, Shi Yan, Ryang Kim, Srivatsa Kothapally, and Leslie Kellam. 2015. “The Consequences of Demographic Matching of Administrative Data.” Poster presented at the APPAM Fall 2015 Conference, Miami, FL, November 12.
Taubman, S. L., H. L. Allen, B. J. Wright, K. Baicker, and A. N. Finkelstein. 2014. “Medicaid Increases Emergency-Department Use: Evidence from Oregon’s Health Insurance Experiment.” Science 343 (6168): 263–68. doi:10.1126/science.1246183.